Staubitz Group
Research
Molecular Switches, New Designs for Dry Adhesion
In the area of molecular switches, we are interested in developing efficient ways of functionalising switchable molecules. Although halogenated azobenzenes typically serve as the electrophilic component in a cross-coupling reaction, it can be advantageous to be able to use them as a nucleophilic cross-coupling partner as well.
For this purpose, we developed a palladium catalysed stannylation protocol, which converts iodinated azobenzenes into the corresponding stannanes in high yields. These can then be subjected to Stille cross-coupling conditions, where the products are obtained in excellent yields.
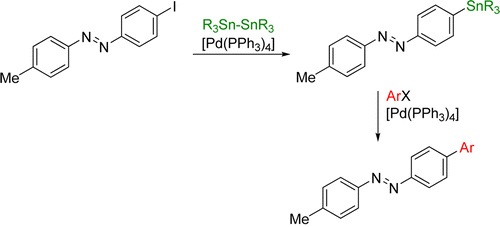
J. Strueben, P. J. Gates, A. Staubitz, J. Org. Chem. 2014, 79, 1719–1728.
A relatively new topic in the area of molecular switches are mechanophoric polymers. We are currently investigating new ways to functionalise the mechanophoric spiropyrans and their uses in materials science.
M. Schulz-Senft, M. Lipfert, A. Staubitz, Chem. Unserer Zeit, 2014 , 48,
200–214.