Staubitz Group
Research
Photoswitches: Azobenzene in Macrocycles
Photochemically switchable molecules and polymers can be reversibly switched back and forth between two or more metastable states with light of a suitable wavelength. We are currently mainly dealing with azobenzene derivatives.1
The incorporation of azobenzene in macrocycles drastically changes the switching properties. In the case of azobenzenes, the Staubitz group investigates macrocycles in which the ring has a significant effect on the thermal half-life of the photoisomer that is energetically higher (Figure 1).2, 3 The aim here is to increase this half-life by means of suitable chemical modifications in such a way that a thermal reverse reaction at room temperature becomes negligible.4 In this way, the aim is to use such a molecule as a store of information that can be both written and erased with light.
As an example from our group, an azobenzene containing ring was prepared, which showed no noticeable thermal reaction after 120 days at 20 °C (the thermal half-life in solution at 70 °C was 24 h) (Figure 1).2
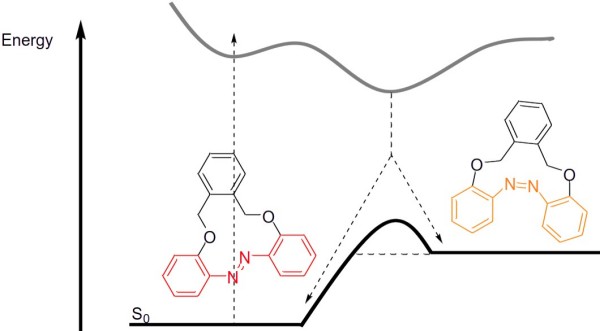
Figure 1: Switching an azobenzene macrocycle with light. The reverse reaction is thermally slow, so that information storage could become possible. (The photoreaction is much more complex than shown here).2
Since it was known in the literature that the fluorination of an azobenzene in the ortho position leads to a considerable extension of the thermal half-life,6, 7 targeted fluorinations were carried out on the starting molecule (Figure 2)4 which led to a considerable extension of the half-life to about 120 years at room temperature.
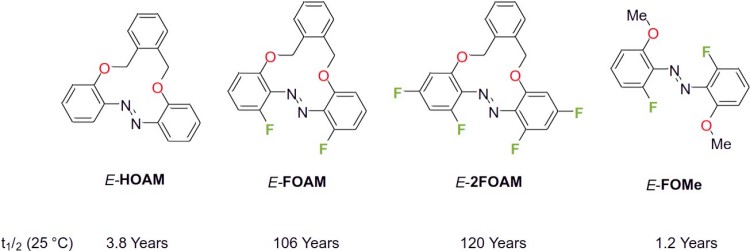
Figure 2: Increasing the thermal half-life time of azobenzenes.4
Most important recent publications:
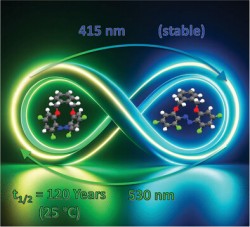
Centennial Isomers: A Unique Fluorinated Azobenzene Macrocyclus with Dual Stability Over 120 YearsS. Schultzke, P. Puylaert, H. Wang, I. Schultzke, J. Gerken, A. Staubitz, Adv. Funct. Mater. 2024, 34, 2313268. Abstract: A macrocyclic azobenzene with unique thermal stability, demonstrating potential for use in optical data storage material is presented: The Z-isomer of this novel photoswitch exhibits unparalleled thermal stability, with a thermal half-life surpassing 120 years at 25 °C. This stability is attributed to the strategic fluorination at two ortho- and both para-positions. Comparative analyses involving its non-fluorinated counterpart, ortho-only-fluorinated variant, and open-chain analog are performed. Employing NMR and UV–vis spectroscopy, X-ray diffraction, alongside Arrhenius, Eyring, and DFT calculations, revealed insights into its extraordinary stability. Furthermore, when incorporated into poly(methylmethacrylate), this material showcase efficient switching with visible light in the solid state, emphasizing its potential for optical data storage applications. |
|
References
(1) Modification of Azobenzenes by Cross-Coupling Reactions.
Walther, M.; Kipke, W.; Schultzke, S.; Ghosh, S.; Staubitz, A., Synthesis 2021, 53, 1213-1228.
DOI.
(2) A new photo switchable azobenzene macrocycle without thermal relaxation at ambient temperature.
Eleya, N.; Ghosh, S.; Lork, E.; Staubitz, A., J. Mater. Chem. C. 2021, 9, 82-87.
DOI.
(3) Synthesis of a Series of 12-Membered Azobenzene Macrocycles and Tuning of the Half-Life of the Thermal Z-E Isomerization.
Ghosh, S.; Eschen, C.; Eleya, N.; Staubitz, A., J. Org. Chem. 2023, 88, 3372-3377.
DOI.
(4) Centennial Isomers: A Unique Fluorinated Azobenzene Macrocyclus with Dual Stability Over 120 Years.
Schultzke, S.; Puylaert, P.; Wang, H.; Schultzke, I.; Gerken, J.; Staubitz, A., Adv. Funct. Mater. 2024, 34, 2313268.
DOI.
(5) Photoelectrochemical information storage using an azobenzene derivative.
Liu, Z. F.; Hashimoto, K.; Fujishima, A., Nature 1990, 347, 658-660.
DOI.
(6) o-Fluoroazobenzenes as Readily Synthesized Photoswitches Offering Nearly Quantitative Two-Way Isomerization with Visible Light.
Bleger, D.; Schwarz, J.; Brouwer, A. M.; Hecht, S., J. Am. Chem. Soc. 2012, 134, 20597-20600.
DOI.
(7) ortho-Fluoroazobenzenes: Visible Light Switches with Very Long-Lived Z Isomers.
Knie, C.; Utecht, M.; Zhao, F.; Kulla, H.; Kovalenko, S.; Brouwer, A. M.; Saalfrank, P.; Hecht, S.; Bleger, D., Chem. Eur. J. 2014, 20, 16492-16501.
DOI.
(8) A Photomechanical Film in which Liquid Crystal Design Shifts the Absorption into the Visible Light
Schultzke, S.; Scheuring, N.; Puylaert, P.; Lehmann, M.; Staubitz, A., Range. Adv. Sci. 2023, 10, 2302692.
DOI.