Staubitz Group
Research
Photoswitches: Azobenzene in Liquid Crystalline Phases
Photochemically switchable molecules and polymers can be reversibly switched back and forth between two or more metastable states with light of a suitable wavelength. We are currently mainly dealing with azobenzene derivatives.1
If azobenzenes are incorporated as mesogens in liquid crystalline phases, the phase transitions can be controlled with light. In the Staubitz group, such films are produced and used in collaborations (Prof. Adelung, Prof. Gorb, University of Kiel) in the field of soft robotics (Figure 3).
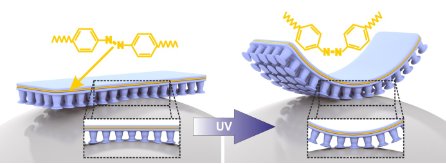
Figure 1: Photomechanical LCEs can be used to produce films that can be bent with light. If these are bonded to a microstructured polysiloxane film, with which dry adhesion is possible, these films can be adhered and de-adhered with light.
The most recent work8 dealt with shifting the switchability into the visible, because on the one hand this reduces the dangers to biological systems and on the other hand makes switching under water possible, since the penetration depth of visible light is significantly higher than that of UV light. For this purpose, a fluorinated derivative was presented (Figure 4) and polymerized.
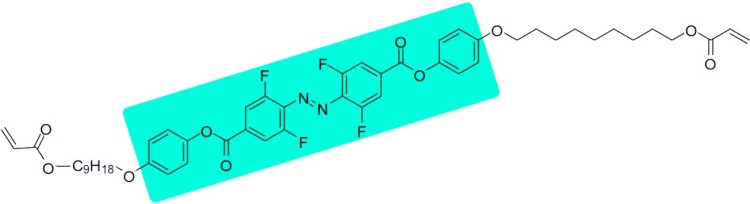
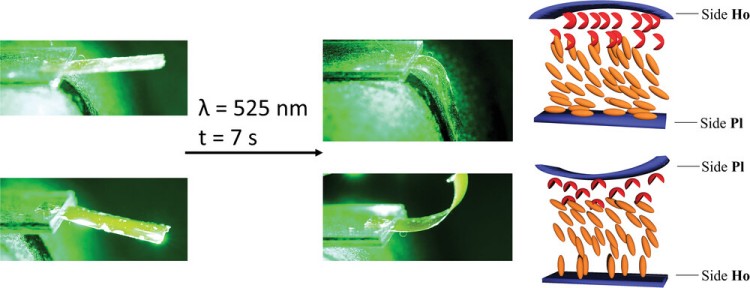
Figure 2: Liquid crystalline monomer that can be polymerized into a photomechanical film. At the bottom left, the mechanistic explanation for the direction of bending of the film: In homeotropic alignment (Ho), isomerization increases the lateral expansion, while in planar alignment (PI) the lateral expansion becomes smaller.8
Most important recent publications:
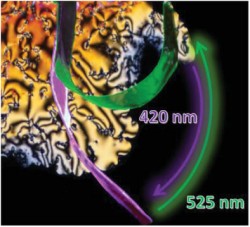
A Photomechanical Film in which Liquid Crystal DesignShifts the Absorption into the Visible Light RangeS. Schultzke, N. Scheuring, P. Puylaert, M. Lehmann, A. Staubitz, Adv. Sci. 2023, 10, 2302692. Abstract: Liquid crystalline polymer networks (LCN) with azobenzene monomers bend reversibly under UV-light irradiation, combining photomechanical and photothermal effects. However, the harmful nature of UV-light limits their use in biology and soft robotics. Although visible light-absorbing tetra-ortho-fluoro-substituted azobenzenes exist, liquid crystalline monomers have never been prepared. Previously, such azobenzenes were added as photoactive additives (up to 10%) to otherwise passive liquid crystalline polymer networks. In this work, a molecular design of a liquid crystalline, polymerizable azobenzene switchable by visible light is presented. The monomer assembles in a highly fluid nematic phase, but polymerizes in a layered smectic C phase. The films are produced solely from the monomer without additional liquid crystalline components and are actuated with visible light. Bending experiments in air and under water differentiate photomechanical and photothermal effects. Remarkably, a 60 µm splay aligned film maintains its deformation for hours, slowly reverting over days. Monomer liquid crystallinity is characterized using differential scanning calorimetry (DSC), wide-angle X-ray scattering (WAXS), and polarized optical microscopy (POM); polymer films are analyzed using WAXS and DSC on a homogeneously aligned film. The synthetic procedure is high yielding and polymer film fabrication is scalable, which enables the use of safe and efficient photomechanical LCNs in soft robotics, engineering and biology. |
|
Bio-inspired Photocontrollable Microstructured Transport DeviceE. Kizilkan, J. Strueben, A. Staubitz, S. N. Gorb, Science Robotics 2017, 2, eaak9454. Abstract: Geckos, which can walk upside down on vertical and underneath horizontal surfaces, owe this ability to the hierarchical structures under their toes. These structures are responsible for substantial adhesion and, at the same time, for quick detachment by mechanical stimulus through leg movements. Inspired by such stimuli-responsive systems in nature, we developed an artificial, photocontrollable microstructured transport device. Through tunable ultraviolet light illumination, the adhesive ability of this bioinspired transport device is reduced up to a factor of 2.7 in terms of adhesive forces and is quickly recovered when the light stimulus ceases. This bioinspired photocontrollable device has been used in a pick-up and drop-down system for transporting planar and three-dimensional solid objects. |
References
(1) Modification of Azobenzenes by Cross-Coupling Reactions.
Walther, M.; Kipke, W.; Schultzke, S.; Ghosh, S.; Staubitz, A., Synthesis 2021, 53, 1213-1228.
DOI.
(2) A new photo switchable azobenzene macrocycle without thermal relaxation at ambient temperature.
Eleya, N.; Ghosh, S.; Lork, E.; Staubitz, A., J. Mater. Chem. C. 2021, 9, 82-87.
DOI.
(3) Synthesis of a Series of 12-Membered Azobenzene Macrocycles and Tuning of the Half-Life of the Thermal Z-E Isomerization.
Ghosh, S.; Eschen, C.; Eleya, N.; Staubitz, A., J. Org. Chem. 2023, 88, 3372-3377.
DOI.
(4) Centennial Isomers: A Unique Fluorinated Azobenzene Macrocyclus with Dual Stability Over 120 Years.
Schultzke, S.; Puylaert, P.; Wang, H.; Schultzke, I.; Gerken, J.; Staubitz, A., Adv. Funct. Mater. 2024, 34, 2313268.
DOI.
(5) Photoelectrochemical information storage using an azobenzene derivative.
Liu, Z. F.; Hashimoto, K.; Fujishima, A., Nature 1990, 347, 658-660.
DOI.
(6) o-Fluoroazobenzenes as Readily Synthesized Photoswitches Offering Nearly Quantitative Two-Way Isomerization with Visible Light.
Bleger, D.; Schwarz, J.; Brouwer, A. M.; Hecht, S., J. Am. Chem. Soc. 2012, 134, 20597-20600.
DOI.
(7) ortho-Fluoroazobenzenes: Visible Light Switches with Very Long-Lived Z Isomers.
Knie, C.; Utecht, M.; Zhao, F.; Kulla, H.; Kovalenko, S.; Brouwer, A. M.; Saalfrank, P.; Hecht, S.; Bleger, D., Chem. Eur. J. 2014, 20, 16492-16501.
DOI.
(8) A Photomechanical Film in which Liquid Crystal Design Shifts the Absorption into the Visible Light
Schultzke, S.; Scheuring, N.; Puylaert, P.; Lehmann, M.; Staubitz, A., Range. Adv. Sci. 2023, 10, 2302692.
DOI.