Staubitz Group
Research
Chemoselective Cross-Coupling Reactions
Transition metal catalyzed reactions for the formation of carbon-carbon bonds are among the most important transformations in synthetic chemistry. Especially the functionalization of aromatic heterocycles is receiving increasing interest since these compounds are ubiquitous in pharmaceutical compounds, dyes or advanced organic materials to name but a few.
While cross-coupling reactions which are selective with respect to the electrophilic site are well researched and understood, reactions that differentiate between two different nucleophilic sites, i.e. different metal or hemi-metal functional groups, are exceedingly rare. In fact, only one such reaction where both sites were located at a single aromatic substrate has ever been reported, but no generality has been demonstrated and experimental procedures and data are all but lacking from that communication.
Developing the synthesis of suitable starting materials and generally applicable methods for their selective transformations are therefore most urgently required.
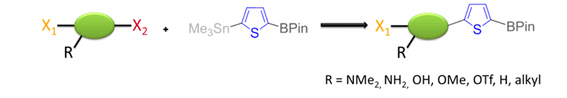
We recently succeeded in developing a new thiophene based dinucleophile, with a stannyl group and a boronic ester. We could show that it was possible to first perform a selective Stille coupling with a variety of electrophiles in very good yields, followed by a Suzuki coupling.

J. Linshoeft, A. C. J. Heinrich, S. A. W. Segler, P. J. Gates, A. Staubitz, Org. Lett. 2012, 14, 5644-5647.
We extended this methodology to a high yielding, both nucleophile and electrophile selective cross-coupling reaction with aromatic rings. The reaction is general with respect to functional groups. Furthermore, the products still contain a boronic ester and a bromide. These two functional groups allow them to be easy-to-prepare, highly complex starting materials for further reactions, avoiding protecting group transformations.