29. Planning a Family
J. S. Leigh, N. Busschaert, C. J. E. Haynes, J. R. Hiscock, K. M Hutchins, L. K. S. von Krbek, A. J. McConnell, A. G. Slater, D. K. Smith, E. R. Draper
Nat. Rev. Chem. 2022, 6, 673-675.
[DOI]
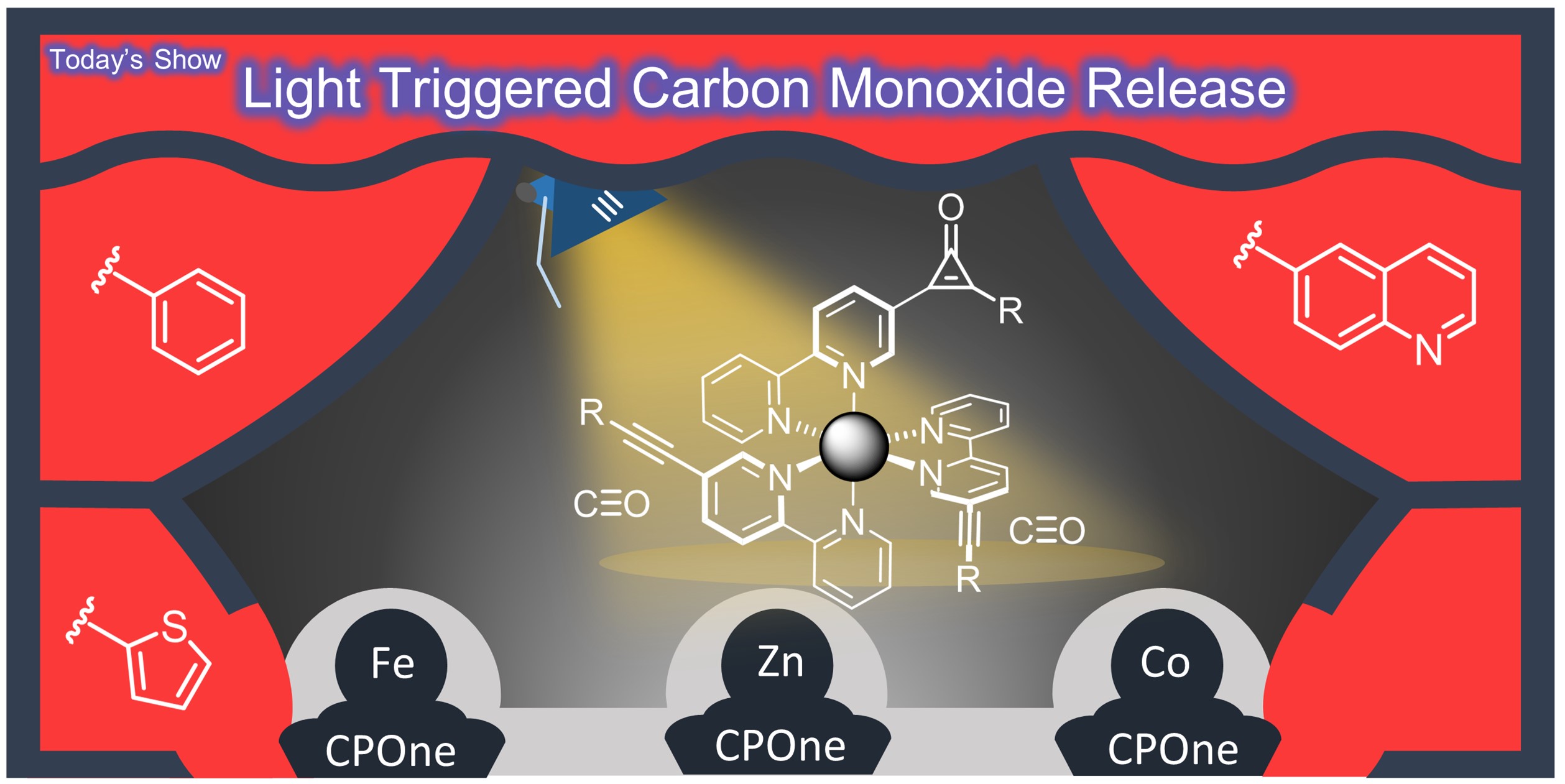
28. M-CPOnes: Transition Metal Complexes with Cyclopropenone-Based Ligands for Light-Triggered Carbon Monoxide Release
M. Lehr, T. Neumann, C. Näther, A. J. McConnell,*
Dalton Trans. 2022, 51, 6936-6943.
Previously available on ChemRxiv https://chemrxiv.org/engage/chemrxiv/article-details/61eed28fc18d673ebe830672
[Abstract]
[DOI]
|
Abstract:
A new class of CO-releasing molecules, M-CPOnes, was prepared combining cyclopropenone-based ligands for CO release with the modular scaffold of transition metal complexes. In proof-of-concept studies, M-CPOnes based on ZnII, FeII and CoII are stable in the dark but undergo efficient light-triggered CO release with the cyclopropenone substituents and metal ions enabling tuning of the photophysical properties. Furthermore, the choice of metal allows the use of different spectroscopic methods to monitor photodecarbonylation from fluorescence spectroscopy to UV/vis spectroscopy and paramagnetic NMR spectroscopy. The modularity of M-CPOnes from the metal ion to the cyclopropenone substitution and potential for further functionalisation of the ligand makes M-CPOnes appealing for tailored functionality in applications. |
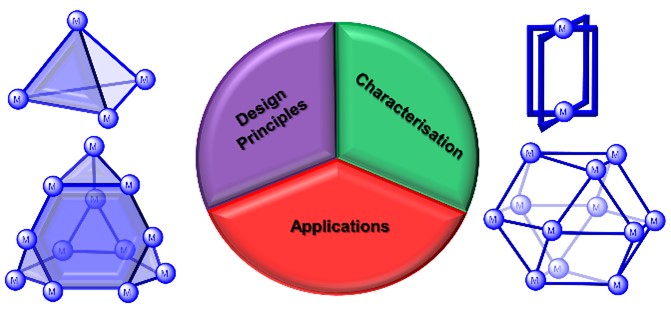
27. Metallosupramolecular Cages: From Design Principles and Characterisation Techniques to Applications
A. J. McConnell,*
Chem. Soc. Rev. 2022, 51, 2957-2971.
Invited article for 2022 Emerging Investigators' Collection
[Abstract]
[DOI]
|
Abstract:
Although metallosupramolecular cages are self-assembled from seemingly simple building blocks, metal ions and organic ligands, architectures of increasingly large size and complexity are accessible and exploited in applications from catalysis to the stabilisation of reactive species. This Tutorial Review gives an introduction to the principles for designing metallosupramolecular cages and highlights advances in the design of large and lower symmetry cages. The characterisation and identification of cages relies on a number of complementary techniques with NMR spectroscopy, mass spectrometry, X-ray crystallography and computational methods being the focus of this review. Finally, examples of cages are discussed where these design principles and characterisation techniques are put into practice for an application or function of the cage. |
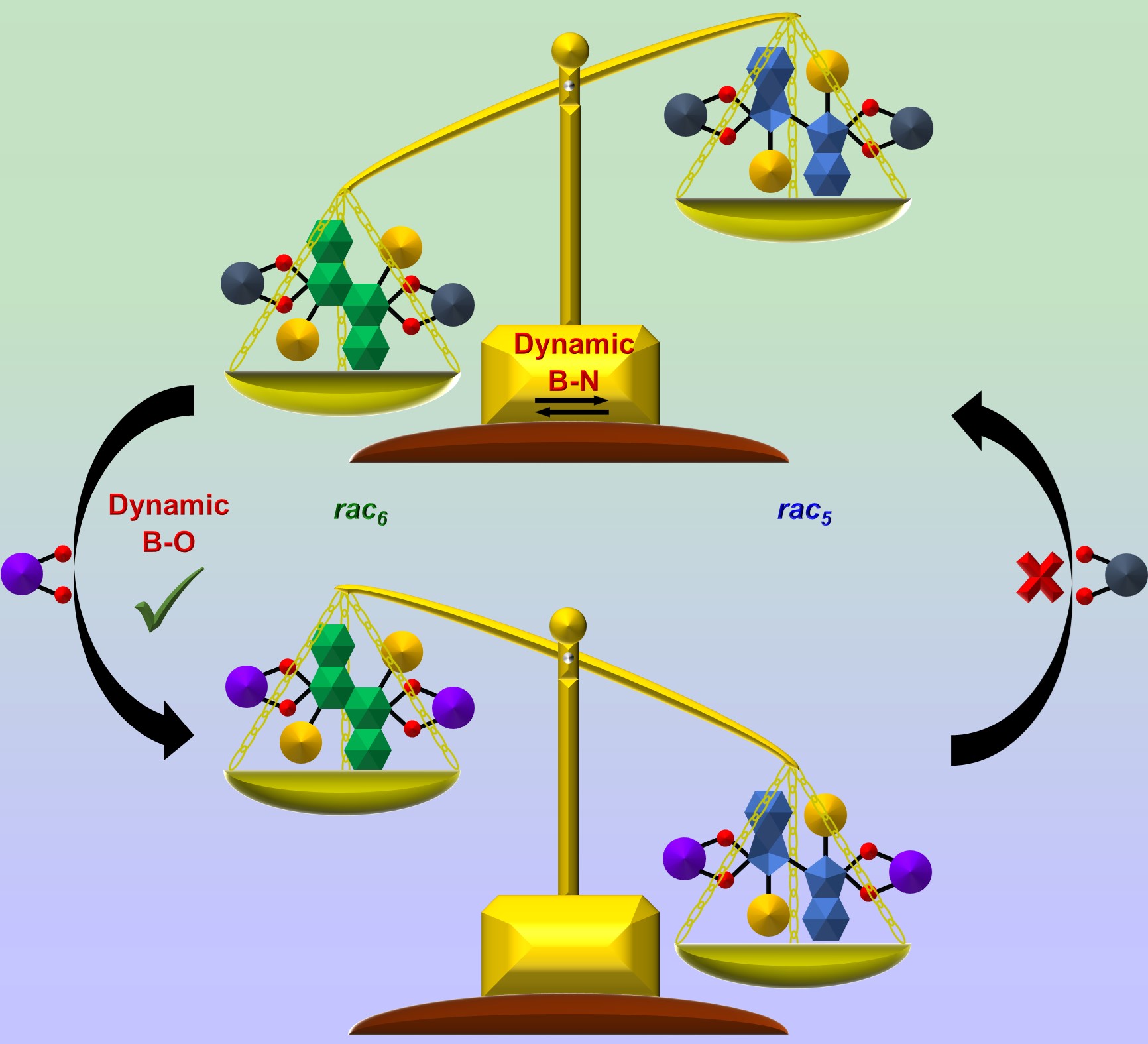
26. The Dynamic Covalent Chemistry of Amidoboronates: Tuning the rac5/rac6 Ratio via the B N and B-O Dynamic Covalent Bonds
P. Harders, T. Griebenow, A. Businski, A. J. Kaus, L. Pietsch, C. Näther, A. J. McConnell,*
ChemPlusChem 2022, e202200022.
Invited article for the 10th Anniversary Special Collection
Highlighted as the Front Cover https://chemistry-europe.onlinelibrary.wiley.com/doi/full/10.1002/cplu.202200084
Previously available on ChemRxiv https://chemrxiv.org/engage/chemrxiv/article-details/61efe72663acbad8ce198a9f
[Abstract]
[DOI]
|
Abstract:
Amidoboronates were prepared as a mixture of up to three isomers (rac5, meso5 and rac6) from the reductive coupling of N-aryl iminoboronates with either cobaltocene or decamethylcobaltocene in acetonitrile. The interconversion of rac5 and rac6 isomers via rearrangement of their dynamic covalent B-N bonds was investigated in solution by redissolving isolated crystals. The aniline para substituent and catechol within the amidoboronates tuned the rac5/rac6; the rac6 isomer predominated for amidoboronates based on pyrocatechol, ranging from a ratio of 0:1 with electron-withdrawing Cl substituents to 0.5:0.5 for electron-donating NMe2 substituents. No interconversion was observed for the rac5 isomers of amidoboronates based on tetrachlorocatechol. Furthermore, the rac5/rac6 distribution was altered by catechol exchange of pyrocatechol for tetrachlorocatechol exploiting the dynamic covalent B-O bonds and the rac5 isomer was the major isomer following exchange. |
25. Supporting the Women That Support the Field of Supramolecular Chemistry
C. Caltagirone, E. R. Draper, J. S. Leigh, C. J. E. Haynes, J. R. Hiscock, A. J. McConnell,
Front. Chem. 2022, DOI: 10.3389/fchem.2022.854085.
Editorial for International Women in Supramolecular Chemistry Research Topic
[DOI]
24. Women in Supramolecular Chemistry- Collectively Crafting the Rhythms of our Work and Lives in STEM
Jennifer Leigh, Jennifer Hiscock, Anna McConnell, Cally Haynes, Claudia Caltagirone, Marion Kieffer, Emily Draper, Anna Slater, Larissa vo Krbek, Kristin Hutchins, Davita Watkins, Nathalie Busschaert 2022, ISBN: 978-1447362371, Policy Press, Bristol.
[DOI]
23. Managing Research Throughout COVID-19: Lived Experiences of Supramolecular Chemists
J. S. Leigh, J. R. Hiscock, S. Koops, A. J. McConnell, C. J. E. Haynes, C. Caltagirone, M. Kieffer, E. R. Draper, A. G. Slater, K. M. Hutchins, D. Watkins, N. Busschaert, L. K. S. von Krbek, K. A. Jolliffe, M. J. Hardie,
Chem 2022, 8, 299-311.
[Abstract]
[DOI]
|
Abstract:
The international Women in Supramolecular Chemistry network believes that taking an area-specific approach effectively supports equality, diversity, and inclusion. Science lacks diversity, and this is intersectional. We share effects of coronavirus disease 2019 (COVID-19) by triangulating findings from an online survey, a collaborative autoethnography, and reflective group research meetings. We show how qualitative research with the community offers insights into challenges and supports individuals, and we demonstrate that research leaders have often taken responsibility for their teams’ mental health and well-being at the cost of their own. |
22. Living Through Covid-19
C. Caltagirone, E. Draper, C. Haynes, C. Hind, J. Hiscock, M. Kieffer, J. Leigh, A. McConnell, A. Slater, L. von Krbek,
Chemistry World 2022.
[DOI]
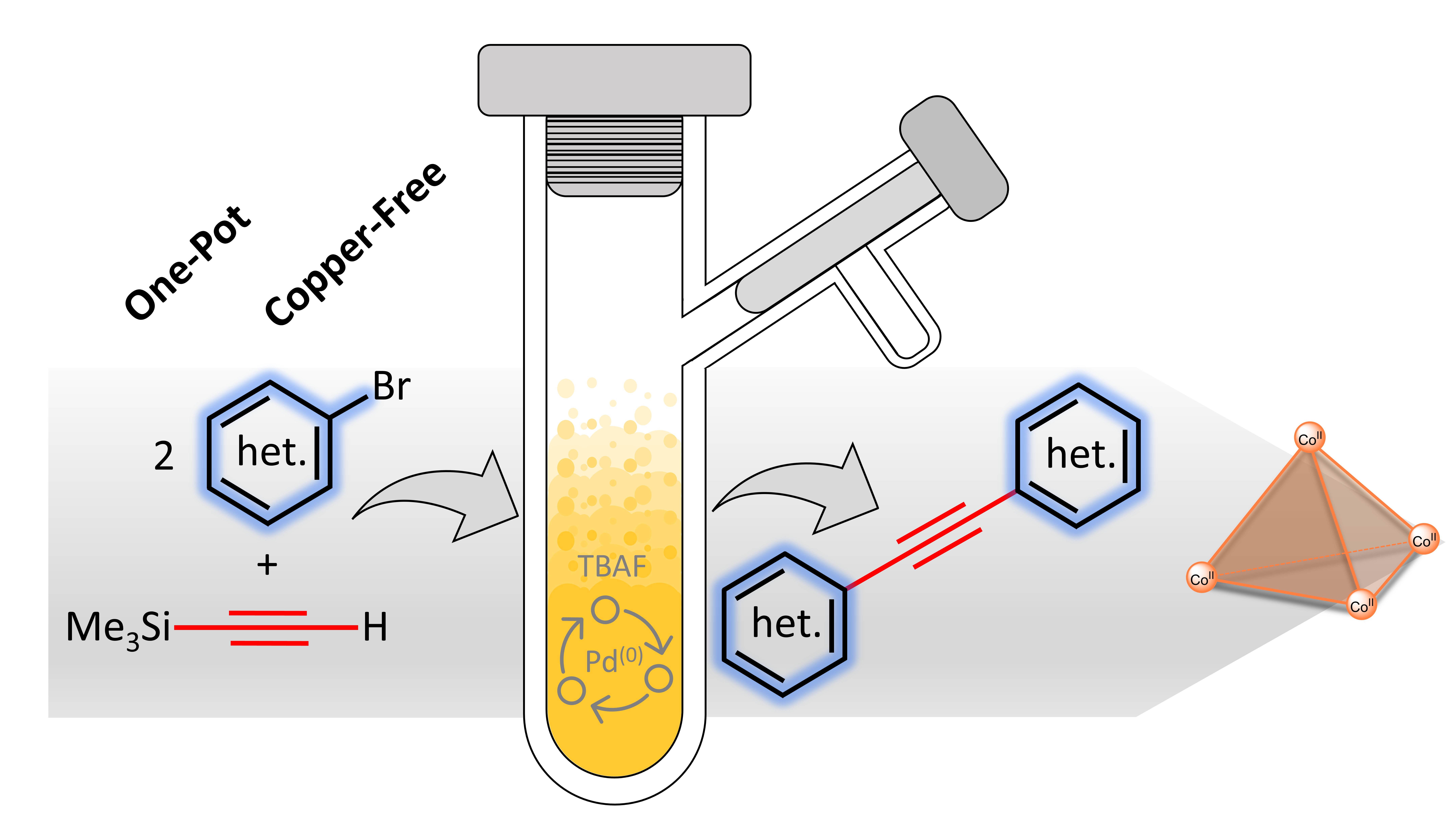
21. Copper-Free One-Pot Sonogashira-Type Coupling for the Efficient Preparation of Diarylalkyne Ligands for Metal-Organic Cages
M. Lehr,† T. Paschelke,† V. Bendt, A. Petersen, L. Pietsch, P. Harders, A. J. McConnell,*
Eur. J. Org. Chem. 2021, 2728-2735.
Highlighted in Chemistry Views https://www.chemistryviews.org/details/ezine/11293778/Symmetric_Diarylalkyne_Ligands_for_MetalOrganic_Cages.html
Highlighted as the Front Cover https://chemistry-europe.onlinelibrary.wiley.com/doi/full/10.1002/ejoc.202100533?af=R
Previously available on ChemRxiv https://doi.org/10.26434/chemrxiv.13625783.v1
[Abstract]
[DOI]
|
Abstract:
Bipyridine- and benzimidazole-based ligands for the self-assembly of Co4L6 cages were synthesised in short reaction times and high isolated yields directly from aryl halide precursors using a copper-free one-pot Sonogashira-type coupling. This one-pot method circumvents the often time-consuming and challenging ligand synthesis for the preparation and application of cages. |
20. An Area-Specific, International Community-Led Approach to Understanding and Addressing Equality, Diversity, and Inclusion Issues within Supramolecular Chemistry
C. Caltagirone, E. R. Draper, M. J. Hardie, C. J. E. Haynes, J. R. Hiscock, K. A. Jolliffe, M. Kieffer, A. J. McConnell, J. S. Leigh,
Angew. Chem. Int. Ed. 2021, 60, 11572-11579.
[Abstract]
[DOI]
|
Abstract:
Diversity, equality, and inclusion (DEI/EDI) are pressing issues in chemistry and the natural sciences. In this Essay we share how an area-specific approach is “calling in” the community so that it can act to address EDI issues, and support those who are marginalised. Women In Supramolecular Chemistry (WISC) is an international network that aims tosupport equality, diversity, and inclusion within supramolecular chemistry. WISC has taken a field-specific approach using qualitative research methods with scientists to identify the support that is needed and the problems the supramolecular community needs to address. Herein, we present survey data from the community which highlight the barriers that are faced by those who take career breaks for any reason, a common example is maternity leave, and the importance of mentoring to aid progression post-PhD. In conclusion, we set out an interdisciplinary and creative approach to addressing EDI issues within supramolecular chemistry. |
19. Calling In Support
C. Caltagirone, E. Draper, M. Hardie, C. Haynes, J. Hiscock, K. Jolliffe, M. Kieffer, J. Leigh, A. McConnell,
Chemistry World 2021.
[DOI]
18. Supramolecular Chemistry: Young Talents and their Mentors
A. J. McConnell,* C. J. E. Haynes,* C. Caltagirone,* J. R. Hiscock,*
ChemPlusChem 2020, 85, 2544-2545.
[Abstract]
[DOI]
Abstract:
Celebrating Supramolecular Chemistry and Mentoring: ChemPlusChem is pleased to publish a Special Collection on Supramolecular Chemistry: Young Talents and their Mentors, guest‐edited by Anna McConnell, Cally Haynes, Claudia Caltagirone, and Jennifer Hiscock. The Special Collection features recent developments in supramolecular chemistry and highlights mentoring relationships between emerging investigators and their mentors.
|
17. From Heteroditopic to Multitopic Receptors for Ion-Pair Recognition: Advances in Receptor Design and Applications
A. J. McConnell,* A. Docker, P. D. Beer,*
ChemPlusChem 2020, 85, 1824-1841.
Very Important Paper in Supramolecular Chemistry: Young Talents and their Mentors Special Collection highlighting mentoring within the supramolecular chemistry community
[Abstract]
[DOI]
Abstract:
Ion-pair recognition has emerged from cation and anion recognition and become a diverse and active field in its own right. The last decade has seen significant advances in receptor design in terms of the types of binding motifs, understanding of cooperativity and increase in complexity from heteroditopic to multitopic receptors. As a result, attention has turned to applying this knowledge to the rational design of ion-pair receptors for applications in salt solubilisation and extraction, membrane transport and sensing. This Review highlights recent progress and developments in the design and applications of heteroditopic and multitopic receptors for ion-pair recognition. |
|
16. A Paramagnetic NMR Spectroscopy Toolbox for the Characterisation of Paramagnetic/Spin-Crossover Complexes and Metal-Organic Cages
M. Lehr, T. Paschelke, E. Trumpf, A.-M. Vogt, C. Näther, F. D. Sönnichsen, A. J. McConnell,*
Angew. Chem. Int. Ed. 2020, 59, 19344-19351.
Previously available on ChemRxiv https://doi.org/10.26434/chemrxiv.12098670.v1
[Abstract]
[DOI]
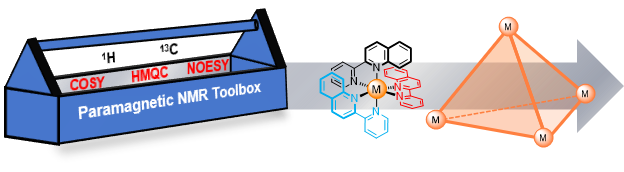
|
Abstract:
The large paramagnetic shifts and short relaxation times resulting from the presence of a paramagnetic centre complicate NMR data acquisition and interpretation in solution. In contrast to the large number of standard NMR methods for diamagnetic compounds, the number of paramagnetic NMR methods is limited and spectral assignment often relies on theoretical models. We report a toolbox of 1D (1H, proton-coupled 13C, selective 1H decoupling 13C, steady-state NOE) and 2D (COSY, NOESY, HMQC) paramagnetic NMR methods for the straightforward structural characterisation of paramagnetic complexes in solution and demonstrate its general applicability for fields from coordination chemistry to spin crossover complexes and supramolecular chemistry through the characterisation of CoII and high-spin FeII mononuclear complexes as well as a Co4L6 cage. The toolbox takes advantage of the reduced signal overlap, decreased instrument time and greater sensitivity from the presence of the paramagnetic centre while overcoming the loss of structural information from the wide chemical shift dispersion and broad signals. In some circumstances, more structural information was revealed in the COSY spectra than would be observable for a diamagnetic analogue; as well as the expected through-bond cross-peaks, through-space and exchange cross-peaks were also observed for mononuclear complexes with multiple ligand environments and fast ligand exchange. With this toolbox, the standard characterisation of paramagnetic complexes and cages is now possible using NMR spectroscopic methods. |
|
15. Coordination Compounds
A. J. McConnell,* M. Lehr,
in Supramolecular Chemistry in Water, Ed. Stefan Kubik, Wiley VCH, 2019, 249-283.
[DOI]
|
14. Orthogonal Stimuli Trigger Self-Assembly and Phase Transfer of FeII4L4 Cages and Cargoes
A. J. McConnell,† C. J. E. Haynes,† A. B. Grommet, C. M. Aitchison, J. Guilleme, S. Mikutis, J. R. Nitschke,
J. Am. Chem. Soc. 2018, 140, 16952-16956.
[Abstract]
[DOI]
|
Abstract:
Two differently protected aldehydes, A and B, were demonstrated to deprotect selectively through the application of light and heat, respectively. In the presence of iron(II) and a triamine, two distinct FeII4L4 cages, 1 and 2 were thus observed to form from the deprotected A and B, respectively. The alkyl tails of B and 2 render them preferentially soluble in cyclopentane, whereas A and 1 remain in acetonitrile. The stimulus applied (either light or heat) thus determines the outcome of self-assembly and dictates whether the cage and its ferrocene cargo remain in acetonitrile, or transport into cyclopentane. Cage self-assembly and cargo transport between phases can in this fashion be programmed using orthogonal stimuli. |
13. Post-Assembly Reductive Coupling of N-Aryl Iminoboronates: Reversible Radical Coupling and Unusual B-N Dynamic Covalent Chemistry
E. N. Keyzer, A. Sava, T. K. Ronson, J. R. Nitschke, A. J. McConnell,* Chem. Eur. J. 2018, 24, 12000-12005.
Part of the 7th EuCheMS Special Issue
[Abstract]
[DOI]
|
Abstract:
Post-assembly reaction of a dynamic covalent iminoboronate system following addition of Cp2Co resulted in the formation of a series of new reductively coupled dianionic dimers via C−C bond formation. The dimers formed as a mixture of BN-containing isomeric products: diastereomers rac5 and meso5, with coupled five-membered rings, and enantiomeric rac6, with a fused six-membered ring bicyclic system from C−C bond formation and rearrangement of the B−N bonds. Each isomer was identified using 1H NMR spectroscopy in combination with single crystal X-ray structure determination. Interestingly, interconversion between the coupled five-membered rings (rac5) and fused bicyclic systems (rac6) was found to occur through an unprecedented breaking and reforming of the B−N covalent bond. Further, the coupled products could be converted quantitatively back to their iminoboronate precursors with addition of the electron abstractor Ph3C+. |
12. Spin-State Switching in Fe(II) Helicates and Cages
A. J. McConnell,* Supramol. Chem. 2018, 30, 858-868.
Part of the ISMSC 2017 Special Issue
[Abstract]
[DOI]
|
Abstract:
Despite the intense interest in helicate and cage supramolecular architectures, only a small fraction
of these are known to switch their spin-state in response to a stimulus, such as temperature and light.
However, these have recently emerged as a promising new class of spin-crossover materials and
although there are still relatively few examples, it is an active area of research. This review discusses
the lessons learned and challenges associated with the design and study of spin-crossover helicates
and cages before highlighting new developments in the field. |
11. Vom Denken und Sprechen
A. J. McConnell,* Nachrichten aus der Chemie 2017, 65, 1034-1035.
[Abstract]
[DOI]
|
Abstract:
Die Wissenschaft kann nicht ohne Sprache existieren, und die Sprache beeinflusst, wie ein Wissenschaftler denkt. Zur Zeit hat Englisch hier die Hauptrolle. Das war nicht immer so und kann sich auch wieder ändern. |
10. A Ruthenium(II) Complex as a Luminescent Probe for DNA Mismatches and Abasic Sites
A. N. Boynton, L. Marcelis, A. J. McConnell, J. K. Barton,
Inorg. Chem. 2017, 56, 8381-8389.
[Abstract]
[DOI]
|
Abstract:
[Ru(bpy)2(BNIQ)]2+ (BNIQ = Benzo[c][1,7]napthyridine-1-isoquinoline), which
incorporates the sterically expansive BNIQ ligand, is a highly selective luminescent probe for
DNA mismatches and abasic sites, possessing a 500-fold higher binding affinity towards these
destabilized regions relative to well-matched base pairs. As a result of this higher binding
affinity, the complex exhibits an enhanced steady-state emission in the presence of DNA
duplexes containing a single base mismatch or abasic site compared to fully well-matched DNA.
Luminescent quenching experiments with Cu(phen)22+ and [Fe(CN)6]3- implicate binding of the
complex to a mismatch from the minor groove via metalloinsertion. The emission response of
the complex to different single base mismatches, binding preferentially to the more destabilized
mismatches, is also consistent with binding by metalloinsertion. This work shows that high
selectivity towards destabilized regions in duplex DNA can in general be achieved through the
rational design of a complex with a sterically expansive aromatic ligand. |
9. Subcomponent Exchange Transforms an FeII4L4 Cage from High- to Low-Spin, Switching Guest Release in a Two-Cage System
A. J. McConnell, C. M. Aitchison, A. B. Grommet, J. R. Nitschke,
J. Am. Chem. Soc. 2017,
139, 6294-6297.
[Abstract]
[DOI]
|
Abstract:
Subcomponent exchange transformed new
high-spin FeII4L4 cage 1 into previously-reported low-spin
FeII4L4 cage 2: 2-formyl-6-methylpyridine was ejected in
favor of the less sterically hindered 2-formylpyridine, with
concomitant high- to low-spin transition of the cage’s FeII
centers. High-spin 1 also reacted more readily with
electron-rich anilines than 2, enabling the design of a
system consisting of two cages that could release their
guests in response to combinations of different stimuli.
The addition of p-anisidine to a mixture of high-spin 1 and
previously-reported low-spin FeII4L6 cage 3 resulted in the
destruction of 1 and the release of its guest. However,
initial addition of 2-formylpyridine to an identical mixture
of 1 and 3 resulted in the transformation of 1 into 2; added
p-anisidine then reacted preferentially with 3 releasing its
guest. The addition of 2-formylpyridine thus modulated
the system’s behavior, fundamentally altering its response
to the subsequent signal p-anisidine. |
8. Dual Stimuli-Induced Formation of an Unusual μ-Hydroxide Bridged [Zn9L5(μ-OH)6]12+ Half Pipe
C. S. Wood, T. K. Ronson, A. J. McConnell, D. A. Roberts, J. R. Nitschke,
Chem. Sci.
2016,
7, 1702-1706.
[Abstract]
[DOI]
|
Abstract:
Low-symmetry metal-organic architectures that feature unusual binding motifs are useful for exploring new modes of guest recognition. Such structures remain difficult to create using current rational design principles. One approach to constructing such architectures is to employ ligands with coordination vectors oriented to preclude the formation of simple, low nuclearity molecular assemblies upon complexation to metal ions. Here we report two new supramolecular assemblies generated from such a ligand: a simple metastable [Zn3L3]6+ assembly, which was observed to convert to a more complex [Zn9L5(μ-OH)6]12+ twisted half-pipe architecture. Two chemically distinct stimuli-an anionic template and a base-must be applied for the conversion to occur. Perchlorate, perrhenate, trifluoromethanesulfonate and 2-naphthalenesulfonate were found to act as competent templates for the [Zn9L5(μ-OH)6]12+ structure. |
7. Stimuli-Responsive Metal-Ligand Assemblies
A. J. McConnell,† C. S. Wood,† P. P. Neelakandan,† J. R. Nitschke,
Chem. Rev.
2015,
115, 7729-7793.
[DOI]
|
6. Luminescence of [Ru(bpy)2(dppz)]2+ Bound to RNA Mismatches
A. J. McConnell, H. Song, J. K. Barton,
Inorg. Chem.
2013,
52, 10131-10136.
[Abstract]
[DOI]
|
Abstract:
The luminescence of rac-[Ru(bpy)2(dppz)]2+ (bpy = 2,2'-bipyridine and dppz = dipyrido[3,2-a:2',3'-c]phenazine) was explored in the presence of RNA oligonucleotides containing a single RNA mismatch (CA and GG) in order to develop a probe for RNA mismatches. While there is minimal luminescence of [Ru(bpy)2(dppz)]2+ in the presence of matched RNA due to weak binding, the luminescence is significantly enhanced in the presence of a single CA mismatch. The luminescence differential between CA mismatched and matched RNA is substantially higher compared to the DNA analogue, and therefore, [Ru(bpy)2(dppz)]2+ appears to be also a sensitive light switch probe for a CA mismatch in duplex RNA. Although the luminescence intensity is lower in the presence of RNA than DNA, Förster resonance energy transfer (FRET) between the donor ruthenium complex and FRET acceptor SYTO 61 is successfully exploited to amplify the luminescence in the presence of the mismatch. Luminescence and quenching studies with sodium iodide suggest that [Ru(bpy)2(dppz)]2+ binds to these mismatches via metalloinsertion from the minor groove. This work provides further evidence that metalloinsertion is a general binding mode of octahedral metal complexes to thermodynamically destabilized mismatches not only in DNA but also in RNA. |
5. Luminescent Properties of Ruthenium(II) Complexes with Sterically Expansive Ligands Bound to DNA Defects
A. J. McConnell, M. H. Lim, E. D. Olmon
, H. Song, E. E. Dervan, J. K. Barton,
Inorg. Chem.
2012,
51, 12511-12520.
[Abstract]
[DOI]
|
Abstract:
A new family of ruthenium(II) complexes with sterically expansive ligands for targeting DNA defects was prepared, and their luminescent responses to base pair mismatches and/or abasic sites were investigated. Design of the complexes sought to combine the mismatch specificity of sterically expansive metalloinsertors, such as [Rh(bpy)2(chrysi)]3+ (chrysi = chrysene-5,6-quinone diimine), and the light switch behavior of [Ru(bpy)2(dppz)]2+ (dppz = dipyrido[3,2-a:2',3'-c]phenazine). In one approach, complexes bearing analogues of chrysi incorporating hydrogen-bonding functionality similar to dppz were synthesized. While the complexes show luminescence only at low temperatures (77 K), competition experiments with [Ru(bpy)2(dppz)]2+ at ambient temperatures reveal that the chrysi derivatives preferentially bind DNA mismatches. In another approach, various substituents were introduced onto the dppz ligand to increase its steric bulk for mismatch binding while maintaining planarity. Steady state luminescence and luminescence lifetime measurements reveal that these dppz derivative complexes behave as DNA "light switches" but that the selectivity in binding and luminescence with mismatched/abasic versus well-matched DNA is not high. In all cases, luminescence depends sensitively upon structural perturbations to the dppz ligand. |
|
4. Heteroditopic Receptors for Ion-Pair Recognition
A. J. McConnell, P. D. Beer,
Angew. Chem. Int. Ed.
2012,
51,
5052-5061.
[Abstract]
[DOI]
|
Abstract:
Ion-pair recognition is a new field of research emerging from cation and anion coordination chemistry. Specific types of heteroditopic receptor designs for ion pairs and the complexity of ion-pair binding are discussed to illustrate key concepts such as cooperativity. The importance of this area of research is reflected by the wide variety of potential applications of ion-pair receptors, including applications as membrane transport and salt solubilization agents and sensors. |
3. Extending the Family of Heteroditopic Calix[4]diquinone Receptors for Cooperative AND Ion-Pair Recognition
A. J. McConnell, C. J. Serpell, P. D. Beer, New J. Chem., 2011,36, 102-112.
[Abstract]
[DOI]
|
Abstract:
New heteroditopic calix[4]diquinone receptors with different anion binding units and calix[4]diquinone scaffolds have been synthesised. Ion-pair binding studies using UV/visible and 1H NMR spectroscopies reveal that receptors 1, 2 and 4 are cooperative AND ion-pair receptors, which display little affinity for 'free' ions but enhanced binding of the ion-pairs NaCl, NH4Cl and KCl. The more preorganised receptor 2 binds the ion-pairs more weakly than receptor 1. Varying the nature of the calix[4]diquinone scaffold appears to have little effect on ion-pair binding, although the calix[4]diquinone framework is more conformationally flexible than the tert-butylcalix[4]diquinone one and can be synthesised using the milder oxidant chlorine dioxide. |
2. Kinetic Studies Exploring the Role of Anion Templation in the Slippage Formation of Rotaxane-Like Structures
A. J. McConnell, P. D. Beer,
Chem. Eur. J. 2011, 17, 1719–1728.
[Abstract]
[DOI]
Abstract:
The first examples of the slippage formation of rotaxane-like structures in the presence of an anion template are reported between a macrocycle, synthesised by exploiting Eglinton coupling, and stoppered pyridinium axle components. The role of the anion template in the slippage process has been explored by kinetic studies. 1H NMR spectroscopic investigations reveal the slippage species formed are not rotaxanes but pseudorotaxanes with some rotaxane character. The anion template significantly influences the amount of rotaxane character and the rate of slippage. Importantly, the fastest slippage rates, kon, are achieved with the non-coordinating hexafluorophosphate anion, whereas the slowest slippage off rates, koff, are observed in the presence of coordinating anions, such as chloride. Since the koff rates are significantly smaller than the kon rates in the presence of coordinating anions, these anions act as templates favouring formation of the slippage species thermodynamically. Consequently, the resulting pseudorotaxanes with coordinating anions have greater rotaxane character. Two strategies for converting the slippage pseudorotaxanes into rotaxanes using hydrogenation or complexation with cobalt carbonyl are investigated. |
1. Calix[4]arene Based Rotaxane Host Systems for Anion Recognition
A. J. McConnell, C. J. Serpell, A. L. Thompson, D. R. Allan, P. D. Beer,
Chem Eur. J. 2010, 16, 1256-1264.
[Abstract]
[DOI]
Abstract:
The synthesis, structure and anion binding properties of the first calix[4]arene-based [2]rotaxane anion host systems are described. Rotaxanes 9⋅Cl and 12⋅Cl, consisting of a calix[4]arene functionalised macrocycle wheel and different pyridinium axle components, are prepared via adaption of an anion templated synthetic strategy to investigate the effect of preorganisation of the interlocked host’s binding cavity on anion binding. Rotaxane 12⋅Cl contains a conformationally flexible pyridinium axle, whereas rotaxane 9⋅Cl incorporates a more preorganised pyridinium axle component. The X-ray crystal structure of 9⋅Cl and solution phase 1H NMR spectroscopy demonstrate the successful interlocking of the calix[4]arene macrocycle and pyridinium axle components in the rotaxane structures. Following removal of the chloride anion template, anion binding studies on the resulting rotaxanes 9⋅PF6 and 12⋅PF6 reveal the importance of preorganisation of the host binding cavity on anion binding. The more preorganised rotaxane 9⋅PF6 is the superior anion host system. The interlocked host cavity is selective for chloride in 1:1 CDCl3/CD3OD and remains selective for chloride and bromide in 10 % aqueous media over the more basic oxoanions. Rotaxane 12⋅PF6 with a relatively conformationally flexible binding cavity is a less effective and discriminating anion host system although the rotaxane still binds halide anions in preference to oxoanions. |
| | | | | |

